What list of basic concepts would be complete without a primer on polar and non-polar molecules?
You’ll recall that chemists live in a world made up of atoms and various assemblies and modifications thereof, which are, in turn, made up of protons, neutrons, and electrons. Protons (which have positive charge and some mass) and neutrons (which are just a squosh more massive than protons) hang out together in the nucleus of your atom, while electrons can be thought of as zipping around the nucleus.
When multiple atoms are part of an assembly in which they are bonded to each other, you have a molecule. For the moment, consider the “bond” between atoms in a molecule to be an electron-sharing arrangement that maintains a certain (average) spatial configuration between the nuclei of the bonded atoms. [1]
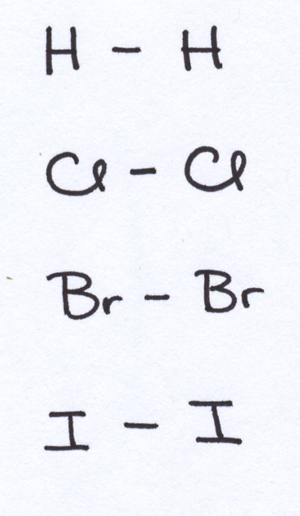 To the extent that the bond hooking the atoms together [2] is made of electrons, a crucial matter is how equally, or unequally, the bonded atoms are sharing these electrons. Some atoms “want” [3] electrons more than others; trends in electron appetite (or electronegativity) are among the many trends which make it useful to keep a Periodic Table of the elements around. I’m not going to describe or rationalize those trends in this post, so you’ll have to trust my representations about which atoms pull at bond electrons with the most enthusiasm.
To the extent that the bond hooking the atoms together [2] is made of electrons, a crucial matter is how equally, or unequally, the bonded atoms are sharing these electrons. Some atoms “want” [3] electrons more than others; trends in electron appetite (or electronegativity) are among the many trends which make it useful to keep a Periodic Table of the elements around. I’m not going to describe or rationalize those trends in this post, so you’ll have to trust my representations about which atoms pull at bond electrons with the most enthusiasm.
The first thing to notice is that whenever you have identical atoms bonded to each other, the electron tug-of-war between them is a tie. In a molecule of H2, the two hydrogens are equally matched; each pulls electrons just as strongly as the other. Thus, the electrons in the bond are equally shared between the two atoms, and the bonding is covalent. The same will be true of Cl2, Br2, I2, O2, N2, and any other diatomic homonuclear molecule (i.e., two-atom molecule with one type of atom).
In situations like these, where the electrons in the bond are shared equally, the bonds are non-polar. This means that no one end of the bond has more or less electron density than the other end.
Not all bonding situations are so egalitarian, however.
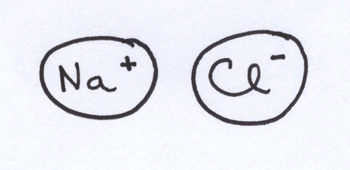 If you bring together two atoms with very different electronegativities, they will not form a bond with any semblance of electron sharing at all. For example, the chlorine atom would sell its own mother[4] to pick up an extra electron of its very own, while the sodium atom would like nothing better than to get rid of one of its electrons. This is why NaCl has no covalent bonds to speak of — Cl rips off an electron from Na to leave us with Na+ and Cl– ions that hang out together due to electrostatic attractions between the two charged ions.
If you bring together two atoms with very different electronegativities, they will not form a bond with any semblance of electron sharing at all. For example, the chlorine atom would sell its own mother[4] to pick up an extra electron of its very own, while the sodium atom would like nothing better than to get rid of one of its electrons. This is why NaCl has no covalent bonds to speak of — Cl rips off an electron from Na to leave us with Na+ and Cl– ions that hang out together due to electrostatic attractions between the two charged ions.
The electrostatic association between Na+ and Cl– ions is often called ionic bonding, even though there’s no “bond” in the sense of shared electrons between the atomic nuclei. Instead of electron sharing here, it’s a “winner take all” situation.
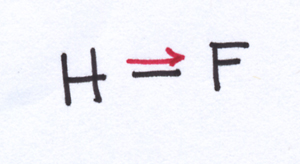 Not every situation that brings together two atoms with different electronegativities results in so lopsided a match. For example, in a molecule of hydrofluoric acid, the difference in electronegativities between H and F is not so large that F walks away with all the electrons. However, F pulls the electrons from the bond more strongly than H does, so that the sharing of electrons between these two nuclei is not equal. As a result, the electrons in the bond spend more of their time hanging out near F than they do hanging out near H.
Not every situation that brings together two atoms with different electronegativities results in so lopsided a match. For example, in a molecule of hydrofluoric acid, the difference in electronegativities between H and F is not so large that F walks away with all the electrons. However, F pulls the electrons from the bond more strongly than H does, so that the sharing of electrons between these two nuclei is not equal. As a result, the electrons in the bond spend more of their time hanging out near F than they do hanging out near H.
When the electrons in the bond are shared, but they aren’t shared equally, what you have is a polar covalent bond.
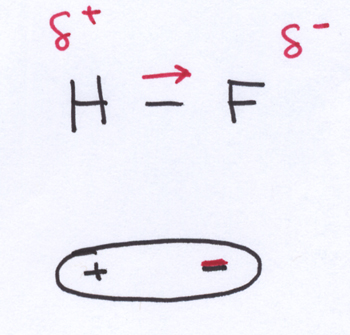 In hydrofluoric acid, the polar covalent bond means that the electron density is unequally spread over the whole molecule. There’s one end of the molecule (the F-end) with a higher electron density, while the other end of the molecule (the H-end) has a lower electron density. Note that HF is a linear molecule, so it only has two ends. The end of the molecule with a higher electron density has a partial negative charge, while the end with a lower electron density has a partial positive charge.
In hydrofluoric acid, the polar covalent bond means that the electron density is unequally spread over the whole molecule. There’s one end of the molecule (the F-end) with a higher electron density, while the other end of the molecule (the H-end) has a lower electron density. Note that HF is a linear molecule, so it only has two ends. The end of the molecule with a higher electron density has a partial negative charge, while the end with a lower electron density has a partial positive charge.
This uneven spread of electron density is what makes HF a polar molecule.
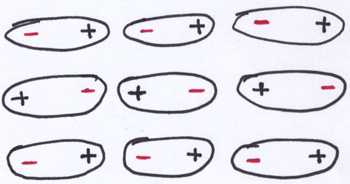 When molecules have positive ends and negative ends, this has some consequences for how they will interact with other molecules. For example, if you have a whole bunch of polar molecules like HF close to each other, they will tend to be arranged in ways that maximize the attractions between opposite charges and minimize the repulsions between like charges. In other words, the positive end of one molecule will tend to hang out with the negative end of another molecule, and so on. (Note that my cartoon version of the happy arrangement of a bunch of HF-like polar molecules is only including two dimensions. Real molecules interact with each other in three dimensions. [5])
When molecules have positive ends and negative ends, this has some consequences for how they will interact with other molecules. For example, if you have a whole bunch of polar molecules like HF close to each other, they will tend to be arranged in ways that maximize the attractions between opposite charges and minimize the repulsions between like charges. In other words, the positive end of one molecule will tend to hang out with the negative end of another molecule, and so on. (Note that my cartoon version of the happy arrangement of a bunch of HF-like polar molecules is only including two dimensions. Real molecules interact with each other in three dimensions. [5])
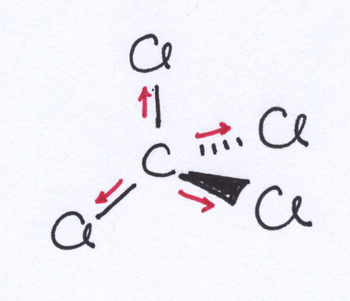 While polar bonds are a necessary ingredient of a polar molecule, they are not a sufficient condition for polar molecules. In carbon tetrachloride, for example, the difference in electronegativity between C and Cl means that each of the four C-Cl bonds is polar, with more electron density being pulled toward the Cl and away from the C. However, the CCl4 molecule has a tetrahedral shape, which means that the partial negative charges on the Cl atoms are distributed pretty symmetrically around the molecule. Meanwhile, the partial positive charge on the C is buried in the center of the molecule. Thus, the CCl4 molecule doesn’t have a positive end or a negative end — instead, despite those nice polar bonds, it’s a non-polar molecule.
While polar bonds are a necessary ingredient of a polar molecule, they are not a sufficient condition for polar molecules. In carbon tetrachloride, for example, the difference in electronegativity between C and Cl means that each of the four C-Cl bonds is polar, with more electron density being pulled toward the Cl and away from the C. However, the CCl4 molecule has a tetrahedral shape, which means that the partial negative charges on the Cl atoms are distributed pretty symmetrically around the molecule. Meanwhile, the partial positive charge on the C is buried in the center of the molecule. Thus, the CCl4 molecule doesn’t have a positive end or a negative end — instead, despite those nice polar bonds, it’s a non-polar molecule.
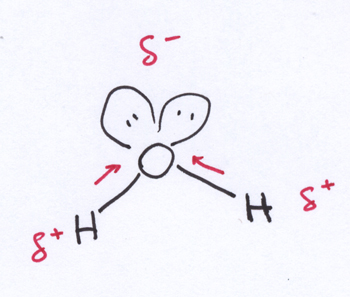 Of course, one of the most famous polar molecules of all time is water, H2O. In this chemist’s cartoon of a water molecule, those things that look like Mickey Mouse ears on the O atom are lone pairs of electrons — pairs of electrons that are associated with the O atom but that are not involved in bonding. Oxygen pulls the bond electrons with more zeal than do the hydrogen atoms, so there’s a delocalization of those electrons away from the H atoms and toward the O atom. This results in a bent molecule shaped almost like a delta kite, with a partial negative charge on the pointy O nose of the kite, and partial positive charges on the H corners at the tips of the wings.
Of course, one of the most famous polar molecules of all time is water, H2O. In this chemist’s cartoon of a water molecule, those things that look like Mickey Mouse ears on the O atom are lone pairs of electrons — pairs of electrons that are associated with the O atom but that are not involved in bonding. Oxygen pulls the bond electrons with more zeal than do the hydrogen atoms, so there’s a delocalization of those electrons away from the H atoms and toward the O atom. This results in a bent molecule shaped almost like a delta kite, with a partial negative charge on the pointy O nose of the kite, and partial positive charges on the H corners at the tips of the wings.
Why is the H2O molecule bent rather than linear? It’s those two lone pairs of electrons sitting on the O atom. They are greedy for space in order to keep their distance from other sources of negative charge on the O (like charges repel and all that). Those other negative bits include the electrons in the H-O bonds. So H2O has an arrangement that’s pretty similar to that of CCl4 — a central atom with four electron-packed thingies arranged around it, each of those thingies needing to be as far from the others as possible. In H2O, it’s just that two of those thingies are lone pairs of electrons.
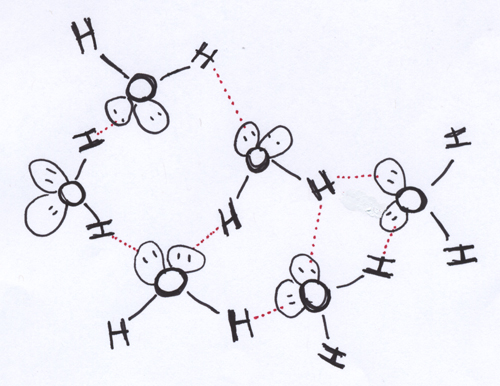
The fact that water, as a polar molecule, looks more like a delta kite than a bar magnet means that arranging a bunch of water molecules in close proximity to each other is a wee bit more complicated. The general idea is the same: you want to put negative ends near positive ends and positive ends near negative ends. Here, though, you have two positive H ends per molecule. Luckily, the partial negative charge on the O atom is big enough that it can hang out with two positive H ends from neighboring water molecules. This results in the chummy intermolecular association represented in the cartoon above.
Understanding the difference between polar and non-polar molecules makes it easier to understand solubility (and insolubility). As well, it’s a useful concept to have under your belt if you want to soothe a cranky baby with a discussion of intermolecular forces, which will be the next basic concept in chemistry I explain here.
_______
[1] The reality is more complicated, but don’t fret about it. You can get a decent understanding of polar and non-polar molecules without attending to all the messy details of what a bond really is.
[2] There aren’t actually hooks. I’m speaking metaphorically. If you listen to an intro chem class, you’ll hear lots of metaphorical talk of this sort.
[3] Talk that is both metaphorical and anthropomorphic! But really, chemists don’t think atoms have desires, or any mental states, for that matter.
[4] Chlorine doesn’t have a mother!
[5] Surfaces complicate things. If they’re thin enough, they may present you with situations in which you’re effectively only dealing with two dimensions.

Thank you. This is being a nice refresher for me, having taken my last chem course 46 years ago.
One question though: How many squoshes in a smidgen? 😉
I should have, but hadn’t, appreciated before now that “covalent” and “ionic” are not two different bonding mechanisms, but the two extremes of a range of possibilities that arise from one bonding mechanism, electron transfer.
Is that a metric smidgen or an Imperial smidgen?
derek: And if you get down to it (if you’re one of us pedantic physical chemistry types) those stick-type bonds don’t exist and there’s just a bunch of nuclei being held together by a nice cloud of electrons.
One of the things I love about chemistry is the way it demonstrates that science is just a set of models, which you improve as you move into more extreme situations.