Some months ago I made a (seemingly idle) threat to follow up my basic concepts posts on polar and non-polar molecules and intermolecular forces with a post on phase changes. Finally it’s here!
Since the discussion here will be leaning on a number of the concepts discusses in the earlier posts, don’t be afraid to click back to them to re-read any of the parts that seem rusty.
First, in this post I’ll only be discussing the three phases of matter taken for granted in intro chemistry classes, namely, solids, liquids, and gases. I won’t be getting into more exotic things like plasmas; there are physicists you can appeal to for the scoop on those. Second, I will not be getting all fancy with possible crystal structures for solids; you might bug a geologist or two for further details there.
What I’ll try to explain in this post are the most basic differences between solid, liquid, and gas phases of a particular substance, and the way that temperature and pressure affect the phase of matter.
Recall that liquids and solids have molecules associated with each other by way of intermolecular forces — forces between molecules. In a solid or liquid, these forces attract individual molecules together — you get “clumps” of the substance. In contrast, substances in gas phase have molecules zipping around, each as if it’s the only molecule in the world, paying no attention to the other molecules that may be around. In the presence of significant intermolecular attractions, those molecules would be paying attention to each other; in the absence of significant intermolecular attractions, what you have in your gas sample is a set of molecules that aren’t clumping.
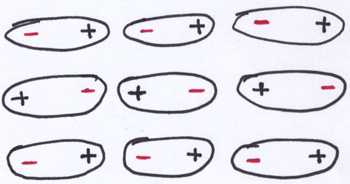
If the gas phase of a substance has approximately no intermolecular attractions holding the molecules to each other, the solid phase of a substance has lots of intermolecular attractions, holding the distinct molecules together in a clump of determinate size and shape that is fairly incompressible. A piston of gas is pretty compressible: you can push down the piston to reduce the volume quite a bit and still have gas in the piston. In contrast, there are limits to how far you can compress a block of iron, a diamond, or an ice cube (although we’ll come back to ice in a moment).
A liquid has more intermolecular attractions holding the molecules to each other than the gas phase, but fewer than the solid phase. As a result, while the resulting clump of molecules is still nowhere near as compressible as the gas phase of the same substance would be, liquids don’t have rigid shapes, and they take on the shape of the containers you pour them into. The intermediate number of intermolecular associations (between gas and solid) means that the molecules in the liquid are sticking together but have some “wiggle room”.
Here, it’s useful to remember that the sort of intermolecular attractions a substance can develop will depends on the polar or non-polar nature of the molecules. Molecules that have distinct negatively charged ends and positively charged ends (like the ones pictured in the cartoon above) will be able to develop stronger attractive forces between molecules than will non-polar substances, which end up associating with each other due to much weaker attractions from synchronized deformations of their electron clouds. It will come as no surprise that weak attractions are easier to disrupt than stronger attractions.
Now, let’s think about pressure and temperature. If you return to the image of gas molecules in a piston, you can think of an increase in pressure as pushing molecules closer to other molecules (because now they’re sharing less space than they were), while a decrease in pressure lets the molecules get farther from each other (because they’re sharing a larger volume). If you start out with a cylinder of gas, you can squeeze your piston (i.e., increase the pressure) to the point where the molecules are close enough to notice each other. When the molecules are close enough to notice each other, what we’re talking about is having the molecules in close enough proximity to develop significant intermolecular forces. Polar molecules don’t need to be quite as close to each other to notice each other (because of those handy negatively and positively charged ends) as do non-polar molecules (which are attracting each other with much weaker fleeting temporary dipoles).
An increase in pressure on a gas can bring the molecules close enough to each other to develop some intermolecular attractions and form a liquid. A further increase in pressure can bring the molecules closer still, generating the additional intermolecular attractions needed to freeze that liquid into a solid — usually. (Water provides an interesting case here; we’ll get back to it.)
How does temperature come into this? The temperature corresponds roughly to the average velocity of the molecules in the system: the higher the temperature, the faster the molecules are moving, the lower the temperature, the slower the molecules.
Now, why does how fast the molecules are moving have anything to do with the phase of matter? The faster the molecules are moving about, the harder it is to get them lined up properly to form good intermolecular attractions. Rather than lining up with the positively charged end of one molecule next to the negatively charged ends of the nearby molecules and maintaining this tidy alignment, the wiggling molecules end up breaking formation, jiggling their positively charged ends close to other positively charged ends (which gives you repulsions rather than attractions) and swinging their negatively charged ends close to other negatively charged ends. So, the higher the temperature, the more likely you are to end up with intermolecular repulsions as well as intermolecular attractions — which means, higher temperatures will favor gases over liquids, and liquids over solids.
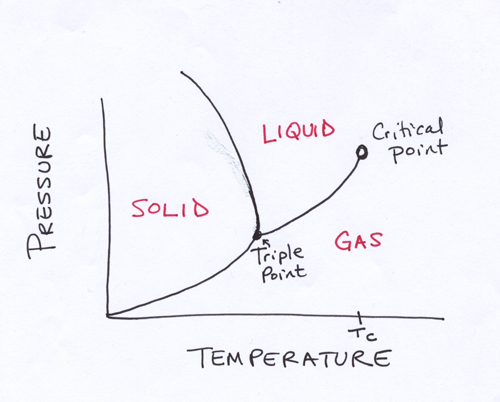
For any given substance, the effect temperature and pressure have on the phase of matter the substance takes can be represented with a phase diagram like the one shown above. You can use a diagram like this to figure out, for any given temperature and pressure, what phase of matter the substance will be in. The lines between the regions marked “SOLID,” “LIQUID,” and “GAS” indicate temperature-pressure combinations where two phases of matter for the substance are in equilibrium — that is, where liquid and solid phases (or liquid and gas phases, or solid and gas phases, depending on which of the lines you’re looking at) of the substance can happily co-exist. The “triple point” where these three lines meet on the phase diagram indicates the temperature and pressure where all three phases of matter of the substance are in equilibrium.
At really high temperatures (above TC, the critical temperature), the average speed of the temperatures is so high that no significant intermolecular attractions can form — even if you squish the molecules really close together by increasing the pressure — and the substance is a gas.
Pick a point in the “GAS” region with a high temperature and mid-range pressure and let’s think about what will happen if we cool it down (moving along a horizontal line from right to left on the phase diagram). Decreasing the temperature (at a moderate pressure, so the molecules are reasonably close to each other) the molecules slow down enough to form some intermolecular attractions. The molecules still move around fast enough that we’ll get some inconvenient intermolecular repulsions, but we’ll have enough intermolecular attractions to put the substance in the liquid phase. Sticking to the moderate pressure and decreasing the temperature even further, the molecules slow down enough to assume the most favorable arrangement of positively charged ends and negatively charged ends — and not to wiggle out of this favorable arrangement (although the molecules will gently vibrate about their places in this arrangement). The large number of intermolecular attractions formed by this choreography results in a solid.
If we had brought down the temperature of the gas phase at a low enough pressure, however, the molecules never would have gotten close enough to each other to develop good intermolecular forces. Instead, the substance would have stayed in gas phase (although the molecules in the gas sample at low temperature zip around more slowly than the molecules in a gas sample at a higher temperature).
You can make the same kind of trip by picking a spot in the “GAS” region, sticking to the same temperature, and gradually increasing the pressure to move upward on the phase diagram. If you’re holding the temperature constant while increasing the pressure, you won’t be changing the average velocities of the molecules, just how close they are to each other. If the spot you started with had a mid to high temperature, the molecules will be jiggling fast enough that you won’t be able to get the perfect alignment of positive ends and negative ends no matter how close together you smush the molecules, and you’ll end up with a liquid. If instead you started at a spot with a low temperature, then smushing the molecules together will transform the gas to a solid, since the slower moving molecules don’t jiggle out of formation and you can develop the attractions needed to maintain the rigid solid arrangement.
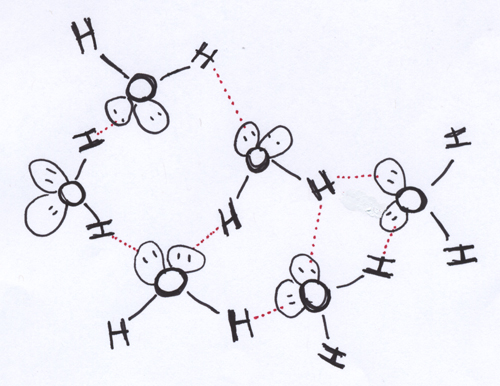
In general, you’d expect the density of the molecules in a substance to be least for the gas phase, greatest for the solid phase, and in between those two extremes for the liquid phase. Water provides an interesting departure from this general trend. Recall that water is a polar molecule with a bent shape, and that it looks more like a delta kite than a bar magnet means that arranging a bunch of water molecules in close proximity to each other is a wee bit more complicated. The general idea is the same: you want to put negative ends near positive ends and positive ends near negative ends. Here, though, you have two positive H ends per molecule. Luckily, the partial negative charge on the O atom is big enough that it can hang out with two positive H ends from neighboring water molecules. This results in the chummy intermolecular association represented in the cartoon above.
In the solid phase of water (you know it as “ice”), the molecules are arranged in three dimensions so that each positive H is near a negative lone pair of electrons on a nearby O, and each lone pair of electrons on an O is near an H. This means that each H2O in the solid phase will be associated (by means of intermolecular attractions) with four other H2O molecules. Achieving this arrangement, with each H2O coordinated with four other H2O molecules, gives an arrangement that looks kind of like a cage with lots of empty space between the adjacent molecules.
In the liquid phase of water, the H2O molecules don’t have that many intermolecular attractions — maybe each H2O is averaging good intermolecular attractions with two other H2O molecules. But because the H2O molecules don’t require such precise arrangements to get intermolecular forces with just two nearby molecules, rather than a tidy cage arrangement, you can have a messier arrangement of H2O molecules with less empty space. Which is to say, the shape of the water molecule means that the arrangement in the liquid phase packs more molecules per unit volume than does the arrangement of molecules in the solid phase. This is another way of saying that ice is less dense than liquid water, and that’s why ice cubes float in a glass of water.

There are a few things that were confusing to me in this area, so i though I would mention them in case they help others.
First, the post are describing the original and common usage of chemical phase changes. Chemists and later physicists who studied this was able to model phase changes. Subsequently physicists discuss phases and phase changes too, as applied to physical systems and as an abstract process.
(Or several processes, for I believe there are at least two fundamental abstract processes – changing phase continuously or discontinuously.)
Second, while I know that the post didn’t mean to discuss exotic phases, but then in all fairness the description “substances in gas phase have molecules zipping around, each as if it’s the only molecule in the world, paying no attention to the other molecules that may be around” isn’t accurate enough.
At everyday atmospheric pressures, molecules collide with each other (and the free surfaces). This gives a friction with surfaces dragged in the gas which is the same as when surfaces are dragged in liquids.
This friction or viscosity explains why gases flows like liquids and mix easily. (Though OTOH it is virtually impossible for two molecules to interact chemically as a gas as opposed to a liquid/solid, or on a helping surface, since momenta and angular momenta doesn’t match.)
Btw, this is why there is a secret phase hidden in your phase diagram. When the pressure becomes low enough (or conversely, the gas volume small enough) the mean free length between molecule collisions becomes larger than the length of the gas volume.
But shh – don’t tell the diagram, it is a secret, remember. The diagram will be messed up. It would take adding a volume axis or something such to straighten it out.
In any case, the gas enters the molecular flow region, where you can for example transport two different gases from different ends of a tube at cross flows and without any significant mixing. They truly doesn’t pay attention to each other.
On Concept
Concept: A basic idea that shapes our perceptions of our universe. In science the most basic concept would be that the universe is understandable. Furthermore, this understanding is teachable, this understanding is improvable, this understanding is imperfect, and this understanding can not be perfected. How and why phase changes occur is neat information, but hardly what I’d call a basic concept.
The idea that matter can take different ‘forms’ is a concept, one that helps a person understand that ice, water, and water vapor are one and the same thing, just mechanically different. What you did in your post is show how the shift from one form to another happens, and what happens when it happens. I recommend re-naming the series “Neat Stuff”, which is what is is.
Alan,
I truly feel for your general sentiment and its application on chemical phases. But in this case it so happens that the abstraction is used to great effect in other parts of science.
For a drastic and grandiose example, when metastable de Sitter false vacua (i.e. those with positive cosmological constant or vacuum energy density, such as ours seems to be) tunnel into a lower energy Minkowski vacua (zero energy/CC) or anti-de Sitter true vacua (negative energy/CC) it is a phase change where a domain wall sweeps the volume at speeds close to light. We will never see the end of our universe before it is upon us.
So for my 2 cents, phase changes are a general and seminal concept that shapes our perception of the universe. Don’t knock processes for forms, because one can solidly argue that processes (such as fields) are what science describes and forms (such as particles) are derivative from those processes.
@Alan
You are being nit-picky about a consistently good post. As you say ‘forms’ of matter is a concept and I’m pretty sure any scientifically trained person would agree that understanding the gas/liquid/solid trio of phases is very basic, therefore it is a basic concept of science. It may not be the most basic (as you point out) but still, I think, should comfortably fall into that category in today’s modern context. Or maybe we could go off on a fun epistemological tangent and assign things to categories like Fundamentally Basic concepts and Secondary Basic concepts and Advanced Basic concepts. Oh the joy of arbitrary categories!
Torbjom Larssen OM,
Still not a concept. It’s a good description of a phenomenon, but it’s not a basic idea, an essntial fact.
That’s what a basic concept is, an essential fact about the universe that governs how you see the universe, and what you can do in it. Inertia is a basic concept, phase change is not. Phase change describes what happens when matter switches from one state to another. Inertia is a fact regarding how the universe works.
Vitis,
Science to be effective needs some clarity of thought and communication. This includes precision in language. To describe a phenomenon we need to have soem agreement on what words mean. This means certain words need to be given a fairly stringent meaning for their use to be effective. “Concept” is one such word. “Phase change” is a useful working term for a phenomenon, but it does not describe an essential fact about the universe. “Inertia” does describe an essential fact about the universe. Thus “inertia” is a basic concept, while “phase change” is not.
Have you ever seen the video of pulling water from a bowl into an inverted glass using a match? How does that little experiment work? Change in pressures? The air pressure in the glass becomes lower than the exterior pressure?
Torbjorn,sorry I don’t know how to make diacritical marks, “This friction or viscosity explains why gases flows like liquids and mix easily. (Though OTOH it is virtually impossible for two molecules to interact chemically as a gas as opposed to a liquid/solid, or on a helping surface, since momenta and angular momenta doesn’t match.)”
This statement makes no sense to me. Does not the chemical interaction known as combustion occur between gaseous oxygen and vaporized gasoline? That is why you get the giant ‘whoosh’ that extends for twenty feet in every direction around the grill when you have to much gas on the charcoal ;P
I’m having a hard time creating a phase diagram.
Help por favor!