As promised last Friday, today we report the results of our investigation of the solubility properties of an avocado. To get the disappointment out of the way up front, we will not be reporting Ks.p. values.

Since we had some around, we decided to use conical tubes to hold the avocado pieces and the experimental solvents. I didn’t want to mark the tubes with Sharpies (because we’ll probably re-use them) and we don’t have the cool colored tape you find in biochemistry labs, so we used a system of plastic cups to keep clear on which tube held which solvent. (The cups also served as our test-tube rack.)

You’ll recall that the three solvents we decided to try were water, vegetable oil, and vodka. (Despite the younger Free-Ride offspring’s suggestion, we didn’t try dissolving avocado in apple juice this time around.)
The water was from the tap.
The oil we used was grapeseed oil (which, as you can see in the picture, has a greenish-yellowish tint to it).
The vodka we used was Trader Joe’s “Vodka of the Gods”.
The avocado we used was perfectly ripe … so much so that we almost didn’t go through with the experiment, because: ripe avocado!
But we are people of science.
We had to find out what we had come to find out. (Also, there were a few more equally appealing avocados in the house that we knew we could eat after our journey of discovery.)

We cut two small chunks of avocado to put into each tube. We did not weigh these avocado pieces (since we were keeping it qualitative for our first foray into avocado solubility), but the amounts of avocado in the three tubes were roughly equivalent. Altogether, the avocado in each tube was about the volume of my thumb.
Before you ask, we have no plans to do solubility tests on human thumbs.
After putting the avocado in each tube, we poured in the solvents to approximately the 32.5 mL mark on each tube.
Here’s the tube with the avocado and water.
The avocado in this tube actually kind of floated. We’ll have more to say about that in a little bit.
The water didn’t change color, and the avocado, while bobbing around in there, didn’t seem to lose any observable volume.

This is the tube with the avocado and the vodka.
The avocado is sitting at the very bottom of the tube.
It did not seem to lose any appreciable volume compared to what we started with.
The vodka looks just as clear as it did in the bottle.
“Is anything going to happen?” asked the younger Free-Ride offspring.
“You have no idea how often scientists ask that question,” I replied.

On to the oil.
Here’s what the tube with the avocado and the grapeseed oil looked like.
“It’s kind of green,” observed the younger Free-Ride offspring. “Is that from avocado dissolving in it?”
“I don’t know,” I replied. “Is it greener than the oil in the bottle?”
“I think it’s a little greener,” ventured the younger Free-Ride offspring.
As in the vodka, the avocado in the tube with the oil stayed at the bottom rather than bobbing up. The edges of the avocado pieces looked a little less sharp than did the edges of the avocado pieces in the other two liquids.
Maybe some of the avocado was slowly dissolving in the oil?
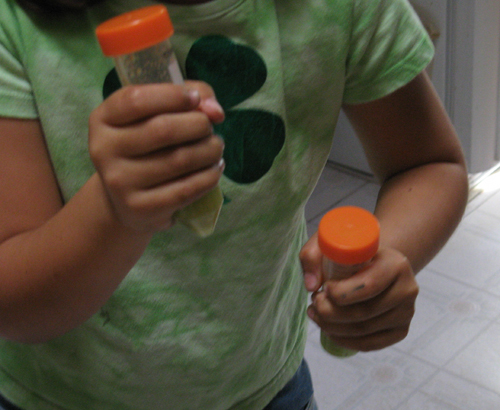
We decided that we should see if we could speed up any dissolving that might be happening by mixing things up a little. Since our kitchen is not equipped with one of those cool vortex thingies that people in biochemistry labs use to mix the contents of their test tubes, we relied upon unpaid child labor to shake up the tubes.
I can’t remember what song was on the radio at the time, but it had a good beat and was easy to dance to.

After the shaking, we let the tubes sit for 15 minutes and then had a look.
Here’s what we saw in the avocado and vodka tube.
The chunks of avocado had definitely gotten smaller in the course of the shaking.
The liquid had definitely taken on a cloudy, greenish cast.
But had we managed to dissolve any of the avocado?
Or had we simply broken it up into itty bitty pieces which were now dispersed in the vodka?

Here’s what we saw in the avocado and grapeseed oil tube after the shaking.
We were actually surprised to see so much of the avocado chunks we started with left intact at the bottom of the tube.
Those avocado chunks did seem to have lost some volume, and they looked increasingly ragged around the edges.
The oil definitely looked greener (and somewhat cloudier).
But it seemed like the avocado and oil tube was more resistant to our shaking.
Hmmmm.

The avocado and water tube was the most surprising.
The shaking seemed to have completely broken up both the avocado chunks we started with.
The liquid in the water was cloudy and bright green.
Obviously, this wasn’t a solution of avocado in water.
Solutions are transparent, and this stuff was opaque.
But something interesting was going on here.
We had to interrupt the experimentation for dinner.
But we decided to let the tubes sit overnight to see what their contents would look like in the morning.
The interval between the shaking of the tubes and the final observation of the tube contents was about 12 hours.

After settling for 12 hours, this is the avocado and vodka tube.
The liquid looks somewhat less green and cloudy than it did shortly after the tube was shaken.
It seems like some of the tiny little avocado bits generated by the shaking settled out, but not all of them.
Maybe some of the greenness of the liquid means we got some avocado to dissolve, but we can’t really tell just by looking at the tube contents.

Here’s what the avocado and oil tube looks like after settling for 12 hours.
The oil is almost as uncloudy as the oil we got out of the bottle. However, it looks maybe a shade greener.
The avocado chunks are still hanging together. Their edges look kind of lacy at this point.
Is the extra green in the liquid due to avocado in solution? Would we have seen the same extra green in the liquid if we had skipped the shaking and just left a couple of chunks of avocado in a tube of grapeseed oil overnight?
(Probably that’s an experimental control we should have run.)

Here’s what what the avocado and water tube looks like after settling for 12 hours.
It actually doesn’t look all that different from what we saw in this tube shortly after shaking it.
Still green, still opaque. No visible sign of settling.
“Did the water dissolve the avocado?” asked the younger Free-Ride offspring.
“This isn’t a solution — it’s still cloudy,” I replied. “But something’s going on here. I didn’t think it would still look like this.”
“Does this have something to do with the avocado floating in the water?” queried the younger Free-Ride offspring.
“That could be,” I answered.
What we know (or think we know):
- Avocado has a relatively high fat content for a fruit.
- Avocado kind of floats in water.
- Oil floats in water.
- If you shake a mixture of oil and water, you can get little bitty droplets of oil dispersed in the water for a while. But eventually, those droplets will coalesce and you’ll end up with a separate layer of oil floating on the water.
- The bits of avocado dispersed in the water after shaking stayed dispersed for a much longer time than we expected.
- Oil did something to the avocado, but it left a relatively large amount of the avocado chunks intact.
- Possibly the viscosity of the oil made our attempts to shake the avocado and oil tube less successful.
- Avocado kind of acts like oil and kind of doesn’t. The components of avocado that aren’t fats may be contributing in important ways to the behavior we observed here.
We can’t really draw any firm conclusions on whether we dissolved any avocado (or avocado components). We think that maybe if we repeat the experiment, filter the liquids to separate out residual avocado chunks, and do a blind taste-test comparison with plain tap water, vodka, and grapeseed oil, we might be able to get some qualitative information on this. (Dr. Free-Ride’s better half seems like the logical taster here.)
One further experiment we’ve decided to try at some future point is to investigate whether we can make mayonnaise substituting mashed avocado for some or all of the oil.
Since Mother’s Day is coming, I’ll wrap up with the list of lab equipment that we’ve decided it would be cool to have in our kitchen:
- The cool colored tape folks use to mark test tubes and other things in biochemistry labs.
- One of those vortex thingies that people in biochemistry labs use to mix the contents of their test tubes.
- A seperatory funnel (and a ring-stand, of course).
- Maybe a distillation apparatus, with which we could try to isolate the distinct chemical components of an avocado.
Not that I’m dropping hints or anything.

Funny, my mom never asked for lab equipment for Mother’s Day. It does sound like a better idea than expensive jewelry, though (or even nonexpensive jewelry).
The components of avocado that aren’t fats may be contributing in important ways to the behavior we observed here.
It seems like there’s a good chance some of those components – possibly proteins – might be acting as an emulsifier, which would explain why the avocado didn’t separate out of the water as you’d expected.
Next time, try if you can phase-separate some of the avocado using a mixture of water and oil. The water and oil layers should separate, and it looks like there would be more avocado in the water layer, but maybe the green stuff would end up only in the oil. And since the water would be the bottom layer, you might not have any big avocado chunks left, and it’s easier to see what the oil does.
Also: try vinegar!
Hmmm. The vodka is probably able to break down the cells, dissolve the chlorophyll and the like. The oil probably isn’t able to break down the cells in the same way.
A solvent extraction would be interesting, although you could only do oil + water or oil + vodka. And how would you separate them with kitchen appliances? Maybe a funnel with plastic wrap (press and seal wrap?) over the bottom? You might avoid the water problem by using green avocado. That way you could see if the emulsion is caused by cells separating, or if it’s actually the cell contents.
This sounds like fun. I think I need to have some children 🙂
What happens if you spin the tubes around? Does the emulsion separate?
You did what with perfectly good vodka???!!
Barbarian.
Shake It Up by The Cars, perhaps?
I really like the idea of an avocado mayonnaise. I think I’d also want a little garlic in it, use lime juice instead of lemon, and add just a wee smidgen (I’m sure a good chemist like Janet knows the precise difference between a smidgen and a wee smidgen.) of cayenne.
Another bit of kitchen chemistry that might want to wait for a few years (unless the sprogs are atypical of their ages and already like hot mustard) is demonstrating the enzyme reaction that releases the hot taste in mustard. If you add water to dried, ground mustard, it will gradually increase in heat over a period of 15-20 minutes. After 20 minutes it will have reached its maximum heat, but you can arrest the increase in heat at any point by adding an acid — either vinegar or white wine works nicely. (Adding dry mustard to a salad dressing, it will not get very hot since the acid is already present, but the fine mustard powder acts as an emulsifying agent and helps keep a vinaigrette in suspension for quite a while.)
First, I have to second Eva’s suggestion of vinegar, that was one of my first thoughts when reading today’s post.
Second,
“If you shake a mixture of oil and water, you can get little bitty droplets of oil dispersed in the water for a while”
Emulsion anyone?
Third, other things you may want to add to your list of equipment (since we’re dreaming):
-Centrifuge
-HPLC …actually, now that I think about it, how about some simple thin-layer chromatography… Mash up some avacado and squish a dab of it on a strip of paper or paper towel and place the edge of the paper into the liquids to see if any of the avacado ‘stuff’ gets pulled along with the capillary action of the liquid.
Got to go!
Scotty B
I second (third?) vinegar for the second trial!
Avocado has both watery and oily substances. So how about water+detergent or vodka+detergent? That way you allow both the water-soluble and oil-soluble components an opportunity to dissolve.
I would be curious what happens to any of the mixtures if you acidify or…shoot, what would be the word? Alkalinize? Basify?…them.
What IS the isoelectric point of guacamole, anyway?…
Finally, I’m going to be disappointed if you don’t get out a microscope to examine the cloudy mixture, and then estimate the volume of liquid which would contain 6.02×10^23 particles (Avocado’s number? One Guaca-mole?)
Be grateful you don’t live in Texas. You could be up for a felony possessing those test tubes. That would be difficult to explain to the sprog and might even put him off science a bit.
Dr. Bill, the dentist who lived across Lincoln St. when I was growing up, used to put “lost” baby teeth in a glass of Coke for a few days to demonstrate how drinking Coke destroys your teeth. I’d be interested to see what (real) Coke does to avocado.
BTW, MOST kids are more partial to peanut butter than avocado, but I know the Sprogs are different.
the list of lab equipment that we’ve decided it would be cool to have in our kitchen
Have always, always, wanted a magnetic stirplate installed on the stovetop. Have not yet envisioned the way to combine the magnetic stirplate with gas heat, however.
You could try filtering the solutions through a coffee filter. Then you could separate out the pieces of avocado from the liquid.
It is cool that you made a colloidal suspension with water and avocado.
You could’ve always labeled the conicals 1, 2, and 3. Generic enough to be used again.
Before you ask, we have no plans to do solubility tests on human thumbs.
Interestingly, the thumb shown in the photos looks significantly hyper-extended enough that it seems categorizable as a hitchiker’s thumb–a recessive genetic trait.
Regarding the effects of water: You’re not working with inert material — “ripe avocado” is living plant tissue!
I suspect that the water-drenched avocado deliquesced, with many of its cells absorbing water until they burst.
The tape we use in the lab only come in boring pastel colors — and often is just masking tape with steam sensitive stripes. Colored electrical tape is much more fun! Bright colors and sometimes even available in grocery stores. We have gotten that at w*rk but that stuff tends to grow legs and walk away.
The cool lab tab is called Fisher Tape (at least, around these parts). But even if you don’t have Fisher tape, you can label conicals with sharpie. A little bit of ethanol will get sharpie off of a conical (or a spec tube, and thanks to my students for that heart attack), and I suspect some rubbing + vodka might do the same.
Pingback: Great Homeschool Links: July 22, 2011
Pingback: Great Homeschool Links: Avocados