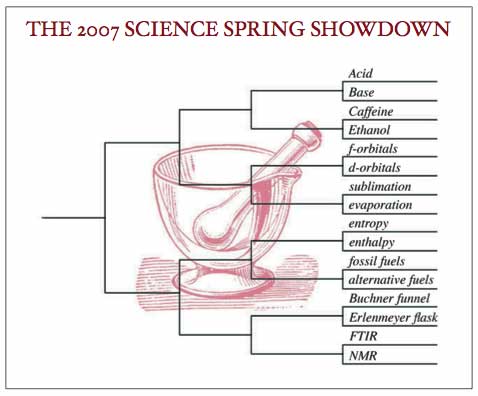
PRESS CENTER | PRINTABLE BRACKETS
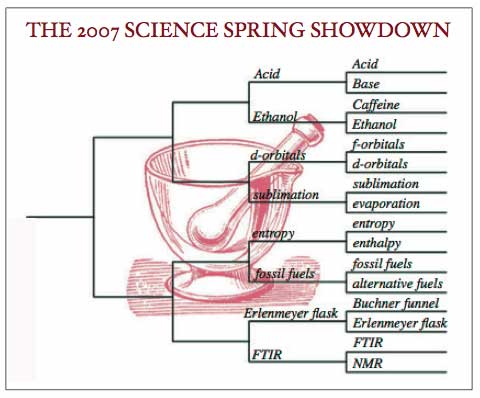
Even given a weekend to come back to equilibrium, some chemistry fans are still perturbed by some of the results of first round play in the MORTAR AND PESTLE bracket. FTIR’s upset win over NMR has many a Monday morning spectroscopist splitting his peaks trying to analyze what went wrong. And while Ethanol is a perennial powerhouse in this conference, many tournament watchers had anticipated celebrating Caffeine at their Monday morning lab meetings.
Friday’s games were just the first step in a mutli-step synthesis of a tournament champion. Tomorrow, just four teams will emerge from the crucible of second round play in the Chemical Arena. The match-ups will be: