PRESS CENTER | PRINTABLE BRACKETS
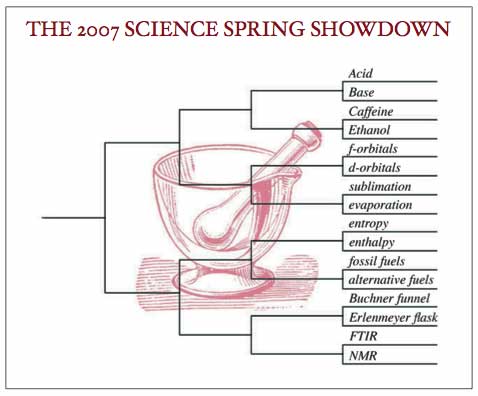
It’s time for a quick run down of the teams from the Chemistry Conference who made it to the tournament this Spring — some who we fully expected to see here, and a few surprises. But it’s also time for you, the fans, to make some noise in support of your favorite teams! If we follow your observations on these competitors down to the quantum level, they’re bound to effect the outcome (albeit in a probabilistic way).
Here are the first round match ups:
