1st ROUND RESULTS | PRESS CENTER | PRINTABLE BRACKETS
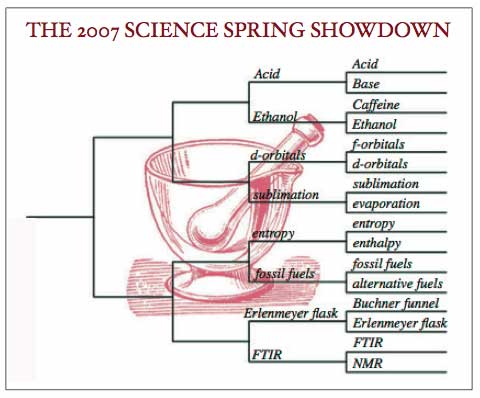
Welcome to coverage of the 2007 Science Spring Showdown second round play in the Chemistry region. The fans in Chemical Arena resorted to a face centered cubic strategy to pack themselves into the stands. You could almost feel the electricity in the air as the products of the first round match-ups were poured into the separatory funnel of the second round. The fans and the teams shook things up. Which teams came out in the top layer, and which saw their hopes of going all the way drained out?
Acid: 105
Ethanol: 87
In one of the most closely watched games of this year’s showdown, the much beloved Ethanol fell to some savvy choices from the Acid coaching staff. Ethanol met its match when butanoic acid took the court. WIth an assist from sulfuric acid, whose concentration on court was remarkable, butanoic acid turned the mighty Ethanol team into a mere ester. The stands got ugly as disappointed Ethanol fans started jeering, “You’ve gone fruity on us! We didn’t come here for girl drinks!” Undeniably, though, the arena smelled of pineapple as the Acid team left, victorious.
Entropy: 98
Fossil fuels: 113
Entropy had a fabulous run this season, but as a state function, how it got here doesn’t matter — just where it started out and where it ended up. Sadly, Entropy won’t be ending up in the tournament finals this year. Early in the game, it looked like Fossil fuels might light a fire that would only increase Entropy’s score. But Fossil fuel’s coaches kept things cool and put the pressure on. Gasoline came onto the court in a high compression engine play that gave the Fossil fuels team just enough extra free energy to take the game and move on to round three.
d-orbitals: 100
Sublimation: 66
This was the game where a repertoire of properties driven by the d-orbitals left Sublimation looking like a team whose intermolecular forces couldn’t hold it together. As Sublimation hunkered down and traded solidity for the vapors, the d-orbitals filled — the fans were dazzled by an astounding range of oxidation states and brilliant colors, not to mention some fancy organometallic footwork. When the d-orbital team maximized its unpaired electrons, they presented a high melting point and a hardness that made them unbeatable. This may be a team in transition (metals), but it’s fair to say it’s a team on the rise.
Erlenmeyer flask: 118
FTIR: 91
The instrumentalists are gnashing their teeth over this upset. Fans had predicted that Erlenmeyer flask might crack under the pressure, but what cracked on the court were FTIR’s cuvettes and KBr pellets. While Erlenmeyer flask held its own, FTIR’s rapid scanning just couldn’t separate signal from noise in this match. In the end, FTIR couldn’t analyze what it was up against, and Erlenmeyer flask captured the win.
This means, going into the Sweet Sixteen, the Chemistry region will see these match-ups in the third round of conference:
Acid vs. d-orbitals
Fossil fuels vs. Erlenmeyer flask
The action is bound to heat up! Stay tuned.

Bracketology, in general, was touted by Bill Geist on CBS Sunday Morning. Bill, of course, had a truly annoying category as his main focus.
http://www.cbsnews.com/stories/2007/03/22/sunday/main2597033.shtml
I am giving him a reference to your post and the Press Room in reply.